Clinical trials are a cornerstone of modern medicine, integral to the discovery and validation of safe and effective medical treatments, drugs, and therapies. However, many people wonder why clinical trials were created in the first place and what roles they serve in advancing healthcare today.
To fully appreciate the significance of clinical trials, it’s essential to explore their origins, evolution, ethical frameworks, and how clinical studies examine the design, implementation, and regulatory oversight of clinical trials. Human clinical trials play a crucial role in assessing the safety, efficacy, and overall impact of medical innovations.
What is Clinical Research?
Clinical research is the systematic study of health and illness in human subjects, aimed at developing new treatments, medications, and medical devices. This field of research is crucial for advancing medical knowledge and improving patient care. Clinical research can be broadly categorized into two main types: observational studies and clinical trials.
Observational studies involve monitoring individuals in their natural settings without intervening. Researchers gather data over time, comparing changes and outcomes to understand the progression of diseases or the impact of various factors on health. These studies provide valuable insights but do not test new treatments directly.
In contrast, clinical trials test medical, surgical, or behavioral interventions in human subjects to determine their safety and effectiveness. These trials are meticulously designed and conducted in phases, each with specific objectives and methodologies. Clinical trials are essential for validating new treatments and ensuring they meet rigorous safety standards before becoming widely available.
By combining observational studies and clinical trials, clinical research offers a comprehensive approach to understanding and improving human health, paving the way for innovative therapies and medical advancements.

The Purpose and Need for Clinical Trials
Before clinical trials became a standard in healthcare, most medical treatments were based on tradition, anecdotal evidence, or untested assumptions. Medicine was a trial-and-error practice, where doctors often relied on treatments passed down over generations without scientific validation. Clinical trials were created to provide a structured, evidence-based approach to testing new therapies, ensuring they were safe and effective before reaching the public. Clinical trial participants play a crucial role in this process, and informed consent along with ethical obligations are paramount to protect their rights and safety. The main reasons behind the creation of clinical trials include:
- Ensuring Patient Safety and Treatment Efficacy: Clinical trials establish a controlled environment to assess how treatments affect patients, identifying potential risks and benefits before they are widely adopted. They also compare new interventions to existing treatments to determine if they are more effective or have fewer side effects.
- Standardizing Medical Treatments: Trials create a unified standard for testing new drugs and therapies, ensuring consistency and reliability in medical research.
- Protecting Patients from Harm: By requiring rigorous testing and safety assessments, clinical trials prevent potentially dangerous treatments from reaching patients without evidence of their safety.
- Advancing Scientific Knowledge: Clinical trials build a robust body of knowledge about diseases, treatment responses, and human biology, contributing to ongoing medical innovation.
A Look Back: The Early History of Clinical Trials
The idea of testing treatments in a controlled, systematic way has roots that extend back several centuries. The first randomized controlled trial, conducted in 1946, tested the efficacy of streptomycin in treating pulmonary tuberculosis. This trial’s meticulous design, including randomization techniques and the use of control groups, set new standards for medical research. Some of the first documented attempts at organized testing of treatments date back to the 18th century. For example:
James Lind’s Scurvy Experiment (1747)
Dr. James Lind conducted what is widely considered the first clinical trial on sailors suffering from scurvy. Lind divided sailors into groups and gave each group a different treatment, ultimately discovering that citrus fruits effectively cured the disease. This pivotal experiment demonstrated that organized testing could lead to medical breakthroughs.
Smallpox and Early Vaccine Trials (1796)
Dr. Edward Jenner’s experiments with smallpox vaccinations in the late 18th century helped establish the practice of inoculation. By observing the effects of cowpox exposure on immunity to smallpox, Jenner pioneered a form of clinical trial that laid the foundation for modern vaccine development.
James Lind and the First Controlled Clinical Trial
James Lind is often credited with conducting the first controlled clinical trial of the modern era. In 1747, while serving as a surgeon in the British Royal Navy, Lind sought to find a cure for scurvy, a debilitating disease that plagued sailors on long voyages. He devised a comparative trial involving 12 sailors suffering from scurvy, dividing them into six groups, each receiving a different treatment.
Lind’s trial included treatments such as cider, vinegar, seawater, and a mixture of garlic, mustard, and horseradish. However, the most promising results came from the group given oranges and lemons. The dramatic improvement in their condition provided compelling evidence that citrus fruits were effective in curing scurvy.
This landmark experiment not only demonstrated the power of controlled testing but also laid the groundwork for modern clinical trials. Lind’s methodical approach to comparing treatments under controlled conditions highlighted the importance of evidence-based medicine, setting a precedent for future clinical research.
The Birth of Modern Clinical Trials: Methodologies and Phase III Clinical Trials
The 19th and 20th centuries marked significant advances in clinical trials, leading to the highly organized and regulated processes used today. The introduction of randomized controlled trials (RCTs) was a breakthrough in clinical research, allowing scientists to minimize bias and increase the reliability of results. RCTs are now considered the “gold standard” in clinical testing, giving researchers confidence that the treatment’s effects can be accurately measured and attributed. Clinical trial protocols are essential documents prepared by expert panels, outlining the scientific rationale, objectives, methodologies, and statistical considerations for the trials. Adherence to standardized guidelines, such as those from the International Conference on Harmonisation (ICH), ensures the safety and effectiveness of trial processes.

Modern clinical trials typically proceed through several phases, each designed to answer specific questions about a treatment’s safety and effectiveness. Gathering preliminary data is a critical part of the trial process, occurring after treatment administration and patient monitoring, and plays a key role in assessing the initial findings of the research. Here’s a look at these phases:
Phase I
Tests the treatment’s safety in a small group of healthy volunteers, primarily to determine side effects and safe dosage ranges.
Phase II
Expands to a larger group to assess effectiveness and further evaluate safety.
Phase III
Involves an even larger group, often in the thousands, to confirm effectiveness, monitor side effects, and compare the treatment to standard options.
Phase IV
Conducted post-approval to monitor the treatment’s performance in real-world settings, assess long-term effects, and detect any rare or long-term adverse effects.
Participant Recruitment and Participation
Participant recruitment and participation are vital components of successful clinical trials. Researchers employ various strategies to recruit participants, including advertisements, online postings, and referrals from healthcare providers. The goal is to find individuals who meet the specific criteria for the study, ensuring that the trial results are relevant and reliable.
Participants in clinical trials are typically patients with the condition being studied. Before joining a trial, they receive detailed information about the study, including its purpose, procedures, potential risks, and benefits. This information is provided through an informed consent form, which participants must sign to indicate their understanding and agreement to take part in the trial.
Throughout the trial, participants are closely monitored to ensure their safety and well-being. They are required to follow the trial protocol, which includes attending scheduled visits, undergoing tests, and adhering to the treatment regimen. In return, participants are often compensated for their time and any expenses incurred during the trial.
The participation of volunteers is crucial for the success of clinical trials, as it enables researchers to gather the necessary data to evaluate new treatments and improve existing ones.

Clinical Trial Protocol
A clinical trial protocol is a comprehensive document that outlines the plan for conducting a clinical trial. It serves as a blueprint for the study, detailing its objectives, design, methodology, statistical considerations, and organizational structure. The protocol ensures that the trial is conducted in a systematic, safe, and ethical manner.
Key components of a clinical trial protocol include the inclusion and exclusion criteria for participants, which define who is eligible to join the study. It also describes the treatment or intervention being tested, the dosage, and the duration of the trial. Additionally, the protocol specifies the outcome measures that will be used to evaluate the trial’s success, such as changes in symptoms, side effects, or overall health improvements.
The protocol is prepared by a panel of experts and undergoes rigorous review and approval by regulatory agencies and ethics committees before the trial can begin. This review process ensures that the trial adheres to ethical standards and regulatory requirements, protecting the rights and safety of participants.
By providing a detailed plan for the trial, the protocol helps researchers conduct clinical trials with consistency and integrity, ultimately contributing to the advancement of medical science.
Ethical Frameworks: Protecting Human Subjects’ Rights and Well-Being
One of the main reasons clinical trials became formalized was to safeguard patient rights. Early medical practices often lacked structured ethical guidelines, sometimes leading to treatments that were neither safe nor effective. The consequences of unethical research practices, such as the infamous Tuskegee Syphilis Study and Nazi medical experiments, highlighted the need for rigorous ethical standards to protect patients. The U.S. Food and Drug Administration (FDA) plays a crucial role in ensuring that clinical trials are conducted according to established protocols to confirm the safety and efficacy of new treatments.
As a response to these ethical violations, several landmark documents and regulations were established:
The Nuremberg Code (1947)
Created in response to Nazi war crimes, the Nuremberg Code established the foundation for ethical clinical research, including informed consent and the obligation to avoid unnecessary harm.
The Declaration of Helsinki (1964)
This set of ethical guidelines from the World Medical Association provides principles for medical researchers, including respect for individuals, the necessity for informed consent, and the requirement to prioritize patient welfare.
The Belmont Report (1979)
Following the Tuskegee Syphilis Study, the Belmont Report introduced principles of respect for persons, beneficence, and justice, reinforcing the ethical obligations of clinical researchers.
Today, these documents and others, like the Good Clinical Practice (GCP) guidelines, govern all aspects of clinical trials, ensuring that participant safety, informed consent, and ethical transparency are at the heart of every study.
Technological Advancements and Clinical Trials: Innovations in Medical Devices
Advancements in technology have revolutionized the way clinical trials are conducted. Digital tools now enable researchers to collect, analyze, and share data more efficiently and accurately than ever before. Some key technological developments include:
- Electronic Health Records (EHRs): These have made it easier for researchers to track patient data, enabling more accurate reporting and monitoring of patient health throughout the trial.
- Remote Monitoring and Virtual Trials: Especially in recent years, virtual clinical trials have gained popularity, allowing participants to take part in studies remotely. This reduces logistical barriers and increases access to a more diverse range of participants.
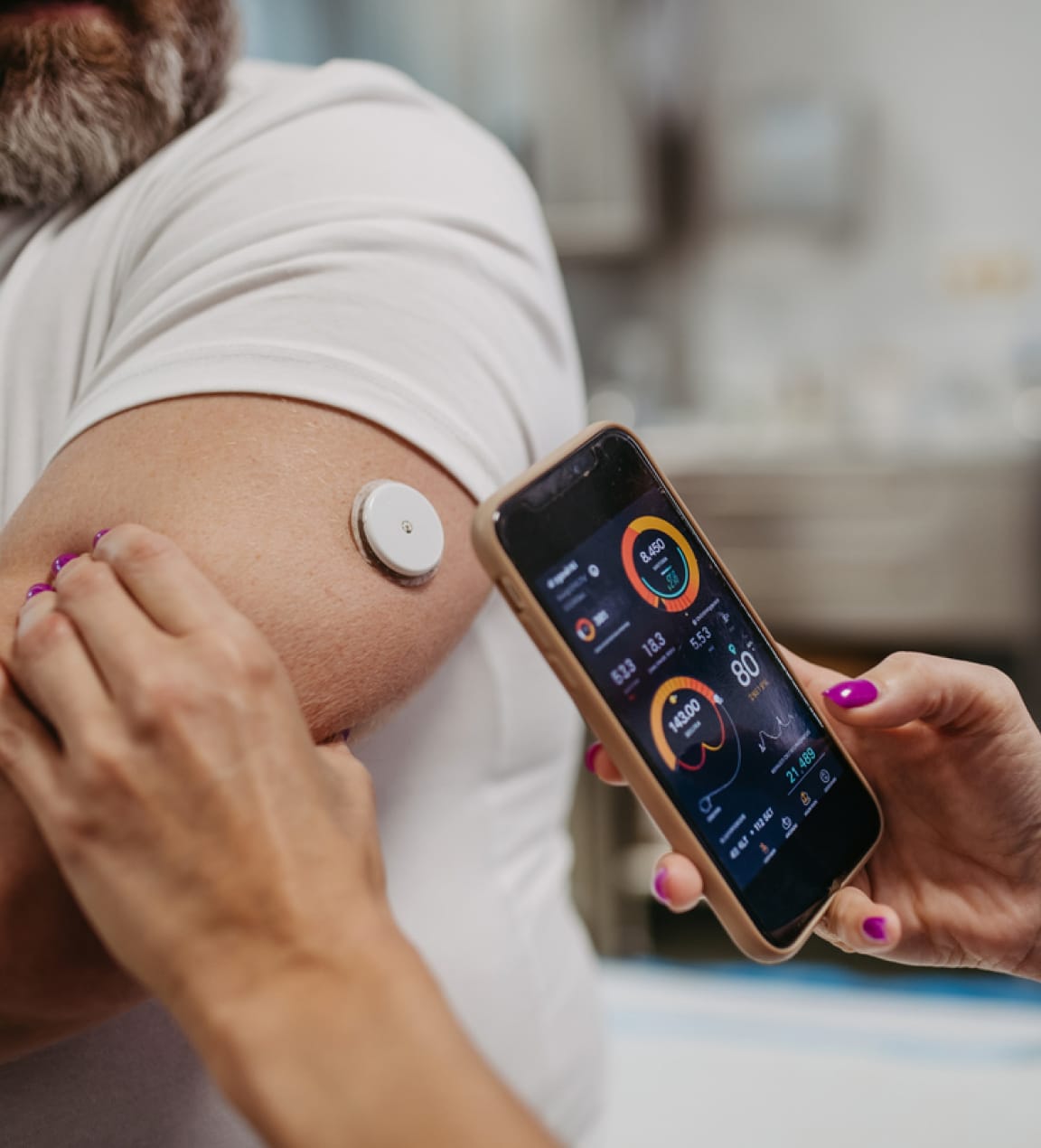
- Wearable Technology: Devices like fitness trackers and heart rate monitors allow researchers to gather real-time data from participants, providing insights into how a treatment may be affecting their daily lives and overall health.
These technologies improve the efficiency, accuracy, and inclusivity of clinical trials, enabling researchers to conduct studies with broader and more representative participant groups.
The Impact of Clinical Trials on Public Health
Clinical trials, including treatment trials that evaluate the effectiveness, safety, and side effects of new therapies, continue to be essential in addressing both common and rare diseases. For example:
Cancer Research
Clinical trials have led to significant advances in oncology, from chemotherapy regimens to targeted therapies and immunotherapy treatments. These studies offer patients access to cutting-edge treatments that may otherwise be unavailable.
Vaccine Development
Clinical trials have proven indispensable in the development of vaccines, including those for diseases like polio, measles, and COVID-19. These trials ensure vaccines are both effective and safe, allowing for widespread immunization against contagious diseases.
Rare Diseases
For rare diseases, clinical trials offer hope for treatments and therapies that might otherwise be uninvestigated. The Orphan Drug Act in the United States incentivizes research on treatments for rare conditions, providing support for clinical trials that benefit small patient populations.
Challenges and Future Directions for Clinical Trials
Despite their importance, clinical trials face challenges, including high costs, complex regulatory requirements, and issues with patient recruitment. Evaluating various surgical procedures is crucial to assess their efficacy, safety, and cost-effectiveness compared to existing treatments. However, several emerging approaches aim to address these hurdles:
- Adaptive Trial Designs: These allow researchers to modify trial parameters based on interim results, potentially saving time and resources while improving data quality.
- Personalized Medicine: Tailoring treatments based on individual genetic profiles is increasingly common in areas like oncology. This approach not only improves treatment efficacy but also reduces the risk of adverse reactions.
- Diversity in Trial Populations: Historically, clinical trials have often lacked diversity, leading to questions about whether treatments are equally effective across all demographic groups. Today, there is a strong push to ensure that trials include participants from varied backgrounds to generate data that reflects the entire population.

Why Clinical Trials Remain Vital
Clinical trials provide a foundational safeguard for public health, enabling the safe and effective introduction of new treatments. Without them, we would lack the rigorous evidence needed to validate new therapies and protect patients. They continue to evolve, leveraging technology and adaptive designs to overcome challenges and deliver results that improve patient outcomes globally.
Join a Clinical Trial to Help Advance Medical Science
Clinical trials were created to establish a scientific, ethical, and structured approach to testing medical treatments, ensuring that new therapies are both safe and effective. From the earliest experiments on scurvy to today’s cutting-edge immunotherapies, clinical trials have evolved to meet the changing needs of medicine.
Today, clinical trials continue to be an indispensable tool in advancing medical science, offering hope and solutions to countless patients worldwide. At M3 Wake Research, our clinical research site network is dedicated to facilitating the latest clinical studies to support ongoing research to help improve patient outcomes for generations to come. Our ongoing commitment to ethical standards and technological innovation ensures that clinical trials will remain a vital force in healthcare for generations to come.
Consider joining a clinical trial at one of our clinics if you’ve recently been diagnosed and are seeking advanced treatments for your condition. Our clinics are regularly enrolling for a variety of indications.

