What We Do
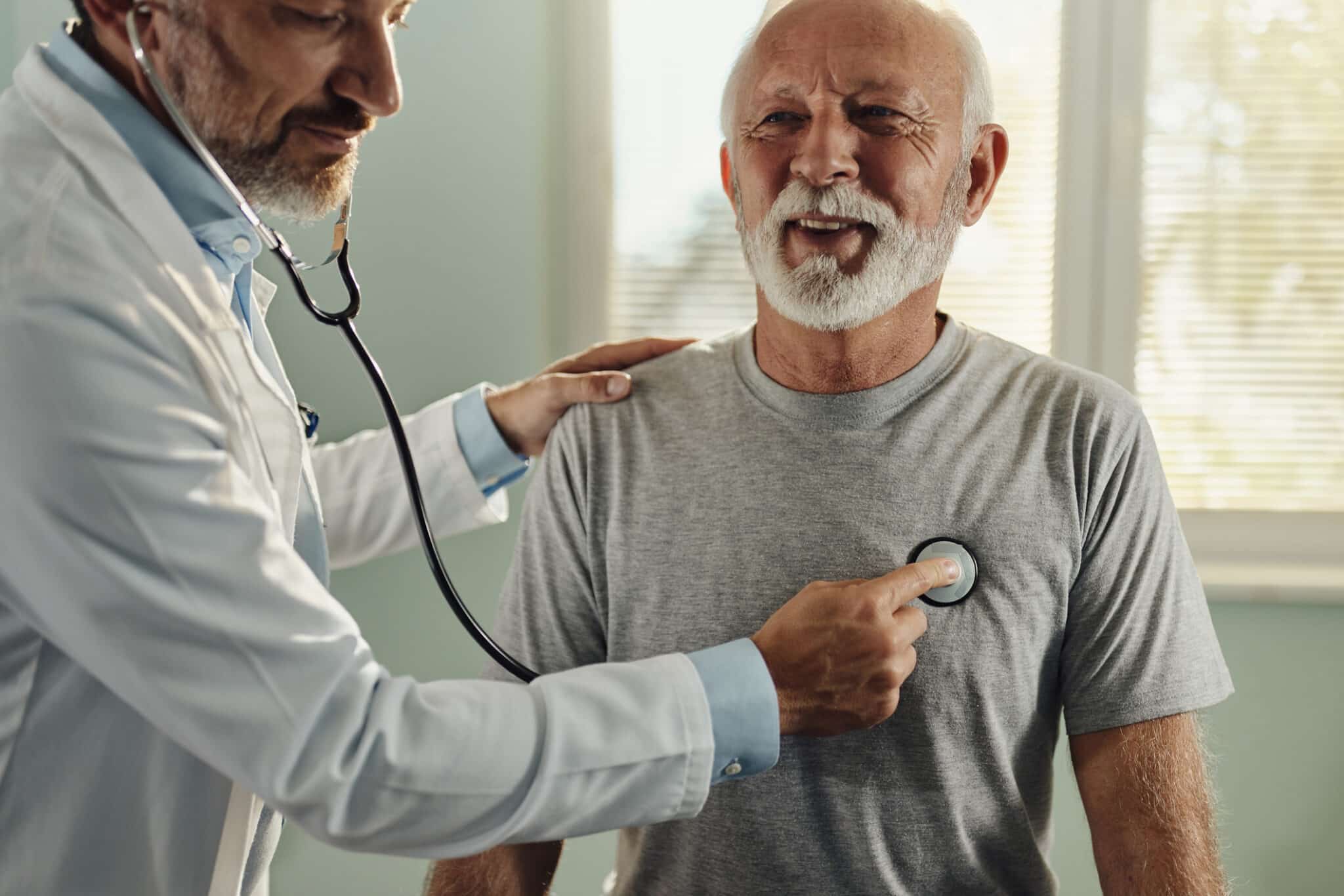
M3 Wake Research is an integrated, wholly owned and operated site organization providing exceptional efficiency in delivering top-tier clinical trial patient care. We are committed to exceeding the expectations of sponsors and CROs through robust and inclusive patient populations from across the country.
We’ve spent over 30 years building meaningful relationships with our partners, patients, and healthcare systems nationwide to offer robust data sources, support systems and information that engage individual participants, including those from minority and underrepresented communities. Your participation matters.
Contributing to 15+ therapeutic areas
Your clinical trial partner in health innovation
Our mission is to improve health by advancing treatments through clinical
research trials delivered with quality, timeliness, and integrity.
Patient-centric, seamless recruitment process
We are committed to exceeding the expectations of sponsors and CROs through robust and inclusive patient populations from across the country. Our Core Values drive our nationwide team to successfully achieve our mission and meet the needs of our Sponsors and CROs:
- We value each other and those we serve.
- We deliver quality.
- We do the right thing.
- We bring our experience and talents to all that we do.
- We are dynamic problem solvers.

Real Partners, Real Experience:
“It has been a pleasure working with your site! I appreciate all of your hard work, dedication to this study and patience. I do hope to work with your site again!” – CRO
Our Nationwide Research Clinics
Our facilities are equipped with the latest testing and research equipment, including the most advanced diagnostic equipment. Select a clinic below to learn more:
The Wake Difference
We are committed to advancing the treatment landscape through seamless clinical trials
Exceptional Trial Experience
for Patients
Participate in clinical trials designed with your needs in mind. We offer patients convenient scheduling, accessible locations, and personalized care plans. Our patient-centered approach ensures that your experience is stress-free, informative, and compassionate.
Comprehensive Support
for CROs
We provide CROs with a seamless integration of personalized patient care, advanced data management, and efficient trial execution, ensuring high-quality outcomes and accelerating the path to medical breakthroughs.
Trusted
Partnership
for HCPs
Healthcare Professionals have access to detailed medical data. Our clinics collect and verify data during clinical trials and report preliminary results to the sponsor. We never share personally identifiable information (PII) and only report consented medical data.

Physicians,
Principal Investigators (PIs), and Key Opinion Leaders (KOLs)
Partner with us to enhance patient experience and healthcare access
Healthcare is a personal choice, and we understand the importance of having a trusted partner in health. We are committed to ensuring the confidence, support, and accessibility you need to support patients navigating unique health challenges.
Our approach is uncompromising – each study conducted is carefully planned and executed according to stringent protocol and regulations with superior quality.
News & Updates
Do you know anyone who would be a good applicant for our studies?